Toyota Kenya develops mechanical ventilator
Automobile assembler Toyota Kenya has developed a mechanical ventilator which can support patients with respiratory failure.
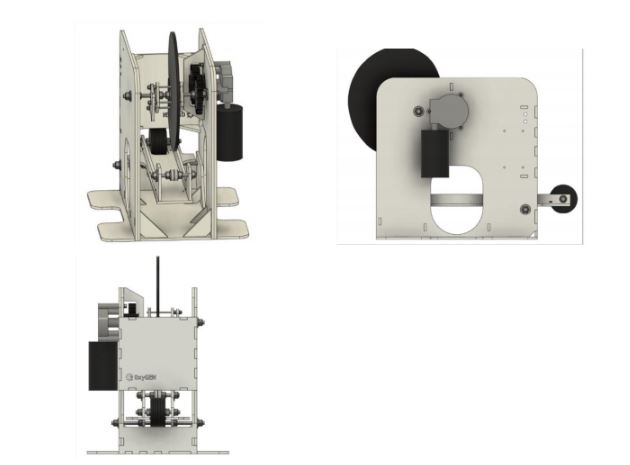
Dubbed “Bridge Mechanical Ventilator” the machine has been designed to enable rapid large-scale development and deployment.
It has been made from locally sourced components which can be easily replicated to avert the COVID-19 pandemic.
According to the Firm Director, Arvinder Reel, the firm has taken up the call by President Uhuru Kenyatta for local companies to come up with easy to deploy local solutions in the battle to contain the spread of the coronavirus.
“It is recognized that the surge in COVID-19 is requiring extraordinary measures that include the provision of mechanical ventilator support to keep pace with clinical need at our healthcare facilities,” he says.
The ventilator comprises of four parts that include: A motor vehicle motor 12V DC, which can be sourced from any car wiper motor, locally fabricated sheet metal pattern which can be cut by CNC machines or fabricated by jua kali artisans, cam pattern which can also be cut from sheet metal plates to control the tidal volume and motor control circuit which can be locally made using available IC, transistor, capacitors, and resistors.
“Once given the necessary approvals, we will be able to develop up to 20 Ventilator per day.”
He reveals that the ventilator makes use of an Ambu bag, which is essentially used for emergencies when a ventilator is not available, where a doctor or a medical practitioner squeezes the bag by hand to push air into the lungs of the patient.
Emergency Ventilator uses this Ambu bag something that is available in abundance in hospitals and squeezes it with the help of a paddle activated by cam discs attached to a mechanical motor.
When in operation, the bridge mechanical ventilator indicates to the operator; the current settings that could include respiratory pressure, tidal volume estimate, frequency; and the current delivery, for instance, inspiratory pressure or respiratory rate.
It is strongly recommended that the device should be used under the supervision of a medical practitioner.















