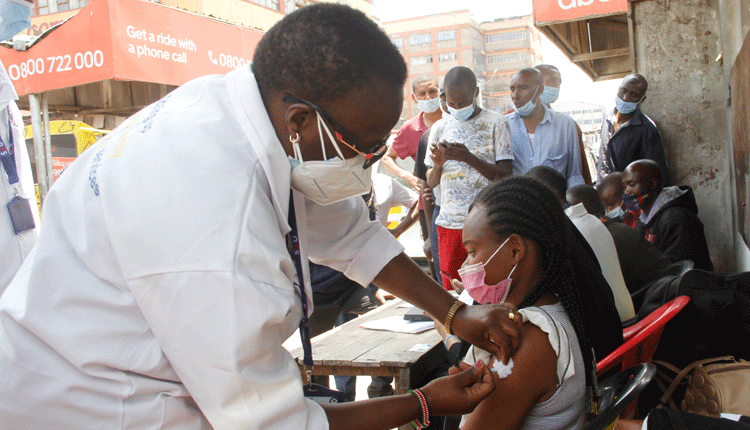Covid-19 vaccine: Demystifying myths, experiences
Like any other African nation, Kenya had waited for the arrival of the Covid-19 vaccine with bated breath.
The vaccine, already being administered in other countries, marked an important milestone in Kenya’s fight against the virus.
Vaccines are needed to prevent infection, severe illness and health crisis.
And so, when Kenya’s acting Director General of Health, Dr Patrick Amoth and others kicked off the country’s vaccination effort by rolling up their sleeves, it signaled hope for a virus that continues to batter the economy.
Kenya received 1.02 million doses of the Oxford-AstraZeneca vaccine, part of an initial allocation to the country of 3.56 million doses.
It was procured by Unicef under the COVAX facility, which is co-led by Gavi (a public-private global health partnership), World Health Organisation (WHO), and the Coalition for Epidemic Preparedness Innovations (CEPI).
It was happening at a time when Covid-19 vaccine hesitancy, safety issues, myths and misconceptions clouded the process.
As expected, with any other form of vaccine, it presented fodder for misinformation.
There were burning questions that needed answers; why did researchers develop Covid-19 vaccine faster within a year than other viruses like HIV? Why did we choose the Oxford vaccine?
Vaccine hesitancy, diminished long queues, nhy,especially among healthcare workers-soon kicked in.
Dr Vincent Muturi-Kioi, a medical director at the International Aids Vaccine Initiative (IAVI) explains that there had been measures in place for pandemics and research work on previous versions of coronaviruses making the pre-clinical analysis for the current coronavirus vaccines much shorter.
There were also overlapping stages of phase studies on the vaccine that allowed for a much faster review of efficacy and safety data.
“A lot of companies also choose to start the manufacturing processes at risk, even before the results became available and this allowed for availability for distribution very shortly after review by the regulatory authorities,” adds Dr Muturi-Kioi.
Unlike SARS-COV-virus, the HIV is a highly variable pathogen. Every time researchers try to get an immune response, the virus mutates.
But the technology used in HIV vaccine, notes the doctor, ”has contributed a great deal in the development of Covid-19 vaccine.”
There are approximately 48 vaccines under clinical evaluation with several showing good safety and immunogenicity (the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal). Eleven of these are currently in phase three of clinical efficacy studies.
To date, seven vaccines are now available for public use, in limited quantities, in dozens of countries. And an estimated 400 million doses have been administered in 132 countries.
But Africa still lags behind in vaccine uptake.
“We have a very long way to go to reach the threshold where we can see the effect of vaccination on the burden of the disease and its spread among the population,” says Dr Muturi-Kioi.
He adds that the initial hope was to have a vaccine with reported efficacy of 50 per cent.
The Oxford-AstraZeneca vaccine, approved by World Health Organisation for emergency use, has over 70 per cent reported efficacy.
Before using vaccine on human beings, it goes through a series of tests, explains the expert.
Typical vaccine
A typical vaccine development includes; pre-clinical analysis, phase I (small numbers of people), phase II (100 participants in the study) and phase III (include 1,000 participants in the study). It is then followed by regulatory review and vaccine approval.
The trials for the Oxford vaccine were evaluated initially in adults in UK, Brazil, South Africa and Kenya. A separate trial was conducted in the US, Chile and Peru.
The United Nations’ health agency found that the AstraZeneca vaccine met the “must-have” criteria for safety, and its efficacy benefits outweighed its risks.
The Oxford-AstraZeneca shot has been hailed because it is cheaper and can be stored at normal fridge temperatures. This makes it easier to distribute.
Doses of AstraZeneca’s vaccine make up the lion’s share of doses in the COVAX coronavirus vaccine sharing initiative.
But the AstraZeneca jab has continued to face safety headwinds, with dozen countries, mostly in Europe, suspending its use for fear the shot may have caused some recipients to develop blood clots.
Few weeks ago, Sweden, Latvia, Germany, Italy, France, Spain, Denmark, Norway and Netherlands had halted the rollout.
Dr Muturi-Kioi notes that, “different people respond differently to drugs and vaccines because of many factors, including human genetics and environmental exposures.”
In assessing report of blood clots from Europe, the European Medicines Agency (EMA) stated that while the investigation is ongoing, the benefits of the AstraZeneca vaccine in preventing Covid-19, with its associated risk of hospitalisation and death, outweigh the risks of side effects.
Dr Amoth, while receiving the jab said the vaccine is safe and free for all.
“Let us potray the correct message to the public,” he said.
And why did Kenya choose the AstraZeneca vaccine, Dr Muturi-Kioi says, “We need to be cognisant that there are not many choices out there.
Although there are many other vaccines, the number of doses is not that many. The cold chain system also fits in well in our healthcare system.”
He further notes the vaccine storage temperature of between two-eight degrees Celsius, “making it fit in well with the vaccine delivery infrastructure that we have in the country making it appropriate for delivery within our setting.”
But he concurs that vaccine hesitancy is not unique to Kenyans and the current situation with the pandemic.
“Even in the 1800s when countries were given mandates for vaccines, there were hesitancy.
It is always related to the information provided and the awareness of the burden of the diseases”
Where do vaccine side effects come from? Experts say the vaccine ‘tricks’ the body into thinking it is fighting coronavirus and tap into our natural immune response to an infection.
Firstly, most of those inoculated, get reaction in the injected arm-swelling and soreness-as the immune system swings into gear.
The Ministry of Health guidelines say those classified as frontline workers and are on government priority list for the jab includes healthcare workers, teachers and the police.
But what do frontline workers who have received the jab think about the whole process?
“As a frontline soldier and the fact that I’m over 60 years, I know I am vulnerable. I have also read a lot about the Covid-19 vaccine.
And so, when the institution informed us about the jab, I did not hesitate,” said Prof Judith Waudo, nutrition lecturer and director of Gender Empowerment and Equity at the Kenyatta University. And the process is simple.
“After filling a form, my temperature was taken, followed by a preamble on who should get the jab.
As soon as I took the jab, I got a serial number and when to receive the second dose through a text message,” she adds.
Professor Waudo further notes the current ‘third wave of the virus’ in the country has claimed some of her immediate relatives making her even more cautious.
“It is an ordinary jab. It comes with some mild side effects such as headache or soreness .In my case, after the being inoculated; the following morning I felt some soreness. But the pain stopped,” she says.
Her second dose will be administered on May 13, and the professor says, “I will still go for the jab. It is better to go with science instead of rumours. As it is, we still don’t have other vaccine options in the country.
I think those who are vulnerable should take the jab. Let us not adopt the ‘last minute rush’ that is Kenyan culture with this jab.”
However, there are those who still won’t take the jab. Kevin Ouma, a boda-boda operator plying Kayole-Umoja route says he is still cautious about taking the jab should it be made available for everybody.
“It is my body, my choice. I think I am still more scared to have the vaccine than not,” added Ouma.
Few days ago, Kentice Tikolo, Managing Director, Cause Impact and a Public Relations a lecturer went for her jab at the Mbagathi Hospital in Nairobi.
Like any other frontline worker, she was over the moon to get vaccinated. My daughter is a doctor and had read a handful of stories about the vaccine.
So when it was her turn to roll up her sleeve at Mbagathi Hospital, it really felt like a moment.
Tikolo explains, “There is no reason being hesitant about vaccines. These jabs have been there for the longest time”
“For me, I am now part of the study. I could have waited for longer, but again, who knows when the vaccine could get to us.”
She also developed some little pain after the jab, which is normal with vaccines.
Side effects
“My hand going downwards is a bit heavy. I have since bought myself painkillers should I feel nauseated because I am expecting a possible side effect,” said Tikolo.
Lack of knowledge about the vaccine is to blame for the low uptake. The health officials didn’t involve everybody in the administration of the vaccine plan.
“The queues and registration process is chaotic. Everybody is now running for the jab, even those who are not in the list, hence the confusion. There should be order,” noted Tikolo.
The reasons for hesitancy over taking the vaccine can be complex-ethnic and based on beliefs.
“As soon as an individual is eligible for vaccination and are asymptomatic, they should be vaccinated,” advises Dr Muturi.