A joint exercise bringing together a number of government agencies in Kenya and Uganda has identified rampant shipment of restricted medicines and dietary supplements known to contain undeclared active pharmaceutical ingredients.
In an unprecedented multilateral initiative bringing focus to the shared threat posed by illicit medical products, it was found that many of these products are used to treat serious or life-threatening medical conditions. During the joint initiative dubbed: Operation African Star, enforcement teams examined shipments of human medicines, biological products, and dietary supplements.
“These examinations revealed several pertinent observations that require synergies to stop.
“These include shipments of medicines which had been stored and shipped outside of approved conditions; prescription medicines lacking valid prescriptions and mis-declared to avoid detection; and dietary supplements known to contain undeclared active pharmaceutical ingredients and/or imported in violation of domestic regulations,” says a joint statement after the teams inspected operations at several points of the two countries.
The teams further observed that these products could potentially be harmful if administered or ineffective when medical treatment is acutely necessary.
“Given Joint taskforce uncovers illegal medicine imports Multi-government agencies in Kenya, Uganda discover a rampant shipment of pills containing undeclared active ingredients that these types of products are often transhipped via third-party countries, they are likely to have been shipped and stored outside of approved conditions, making it difficult to determine if they are safe and effective if obtained from outside the regulated pharmaceutical supply chain,” the teams also bringing together multilateral partners in the US Food and Drug Author[1]ity, the UK, European Union among others said.
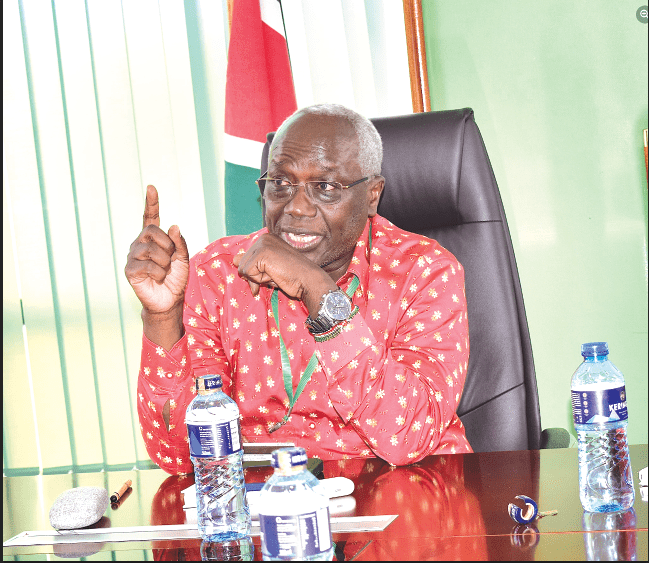
Illicit trade Speaking during the meeting, Chief Ex[1]ecutive Officer of the PPB, Dr Fred Siyoi, said, the threat posed by illicit trade in health products and technologies impacts global public health, and consumers.
“This risk increases when health products and technologies are obtained outside of the unregulated supply chain,” he added, noting that safety and efficacy which cannot be assured threatens the East African Community and public health at large.
Dr Siyoi said that the PPB is committed to protection of public health through collaboration with domestic and interna[1]tional partner agencies and organisations.
“PPB is proud to partner with the NDA to design and execute Operation African Star. We envision this as an iterative initiative with the potential to develop into an internationally recognised best practice,” he said.
The Chairman of the National Drug Authority (NDA) of Uganda, Dr Medard Bitekyerezo, said, a multilateral initiative like Operation African Star allows partners to exchange and develop best practices while positioning themselves to collectivel combat current threats to pub.